We all know that our blood is responsible for delivering oxygen and other important nutrients to our cells, but what you may not know is that the acidity or alkalinity of your blood can also affect your health. Blood with a high acidity level can cause problems such as heart disease, osteoporosis, tooth decay and other oral problems. On the other hand, blood with a low acidity level can have the opposite effect.
Excess body acidity is thought to be the first step toward premature aging, vision and memory problems, wrinkling, age spots and hormone system dysfunction, along with other age-related issues. Our bodies tend to become more acidic as we get older. Most elderly people are highly acidic, with toxic wastes accumulating in the bloodstream, cells, and lymphatic system. Acidic wastes come from different sources; however, it is possible to dramatically slow down the aging process if you kept your skin, muscles, organs, and glands in good PH balance, like they were when you were a baby.

Why pH in the Blood is Important
The pH level in your blood is one of the most important indicators of health. It’s a measure of the alkaline or acidic levels in the body and it provides an indication as to what’s happening inside your cells. The body needs to maintain a balance between acidity and alkalinity to function properly and at its best. In fact, it’s so important that if it falls out of balance, it can cause a number of unnecessary health problems such as fatigue, joint pain, bone loss, kidney stones and even cancer. Unfortunately, many people don’t realize the importance of keeping their pH levels in check.

What Causes Blood Acidity
The reason is actually quite simple; without oxygen, glucose undergoing fermentation and breakdown becomes lactic acid. This causes the pH of the cells to drop to 7.0. There are two types of acidosis, each with various causes. The type of acidosis is categorized as either respiratory acidosis (when the lungs do not expel enough carbon dioxide) or metabolic acidosis (when you ingest substances that contain or produce acid).
Respiratory Acidosis
Respiratory acidosis occurs when too much carbon dioxide builds up in the body. Normally, the lungs remove Carbon Dioxide while you breathe. We breathe in oxygen and exhale carbon dioxide to help get rid of waste. However, sometimes your body can’t get rid of enough carbon dioxide. Here is why.
Main Causes of Respiratory Acidosis Include:
- Chronic airway conditions, like asthma
- Injuries to the chest
- Obesity, which can make breathing difficult
- Sedative misuse
- Overuse of alcohol
- Muscle weakness in the chest
- Problems with the nervous system
- Deformed chest structure

Metabolic Acidosis
Metabolic acidosis starts in the kidneys instead of the lungs. The kidneys play an important role in keeping our bodies in balance by removing waste, toxins and excess water from the blood stream, which is then eliminated through our urine. Acidosis (the condition of having too much acid in the body fluids) occurs when your kidneys can’t eliminate enough acid or when they get rid of too much base (alkalosis).
There are 4 Major Forms of Metabolic Acidosis:
- Diabetic Acidosis occurs in people with diabetes that is poorly controlled. If your body lacks enough insulin, ketones build up in your body and acidify your blood.
- Hyperchloremic Acidosis results from a loss of sodium bicarbonate. This base helps to keep the blood neutral. Both diarrhea and vomiting can cause this type of acidosis.
- Lactic Acidosis occurs when there’s too much lactic acid in your body. Causes can include chronic alcohol use, heart failure, cancer, seizures, liver failure, prolonged lack of oxygen, and low blood sugar. Even prolonged exercise and extreme sweating can lead to lactic acid buildup.
- Renal Tubular Acidosis occurs when the kidneys are unable to excrete acids into the urine. This causes the blood to become acidic.

Signs and Symptoms of Acidosis
As we age, our blood acidity gradually increases. Even though it may seem like a minor change, this increase in acidity can have some pretty major consequences for our overall health. A lot of people experience acidosis and may not even realize it. Watch for these major signs and symptoms:
Initial Signs and symptoms of Acidosis:
- Feeling weak, tired, and having low energy
- Experiencing agitation, anxiety, panic attacks and depression
- Having skin problems like eczema, psoriasis, acne and hives
- Suffering generalized aches and pain
- Experiencing diarrhea, constipation, or bloating
- Suffering from cramping before or during periods
- Experiencing heartburn
- Needing more sleep
- Having increased dental decay
- Feeling nauseous
- Suffering from loss of libido

Chronic & More Serious Signs and Symptoms of Acidosis:
- Osteoporosis
- Weak immune system
- Chronic digestive problems
- Arthritis, joint and ligament problems
- Kidney stones, kidney diseases and gout
- Heart and circulation problems
- Fungal and bacterial infections
- Cancers
- Acidosis

How to Check Your Body’s pH Level
Your body works constantly to carefully control pH levels of blood and other fluids. The body’s pH balance is also called the acid-base or acid-alkaline balance. The good news is, there are many ways to measure your pH levels and determine what the results may mean for your overall health. The term “pH” stands for potential of hydrogen, which is a measurement of how acidic or alkaline something is on a scale from 0-14. The readings are based around a pH of 7, which is considered neutral, like pure water:
- A pH below 7 is acidic.
- A pH higher than 7 is alkaline or basic.
This scale might seem small, but each level is 10 times bigger than the next. For example, a pH of 9 is 10 times more alkaline than a pH of 8. A pH of 2 is 10 times more acidic than a pH of 3, and 100 times more acidic than a reading of 4.
What is Considered Normal Blood pH?
Your blood has a normal pH range of 7.35 to 7.45. This means that blood is naturally slightly alkaline or basic. In comparison, your stomach acid has a pH of around 1.5 to 3.5. This makes it acidic. A low pH, or higher acidity that ranges in some areas of the digestive track and stomach is responsible for digesting food and destroying any germs that get into the stomach.
Types of Tests to Measure PH Levels in the Body
Testing Blood pH
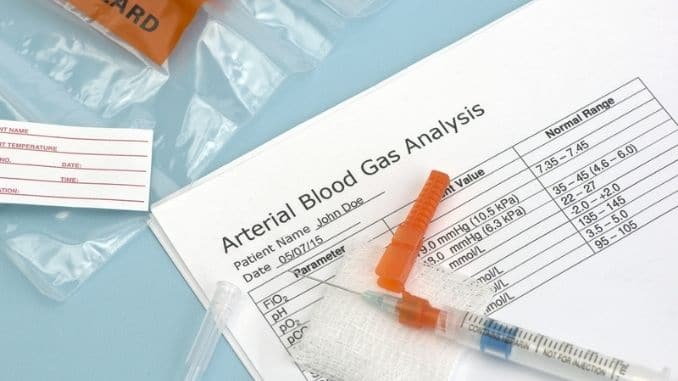
A blood pH test is a normal part of a blood gas test or arterial blood gas (ABG) test. It measures how much oxygen and carbon dioxide is in your blood.
Your doctor might test your blood pH as part of a regular health checkup or if you have a health condition.
Blood pH tests involve having your blood drawn with a needle. The blood sample is then sent to a lab to be tested.
Home Kit Blood Finger-prick Test
An at-home blood finger-prick test is a convenient and easy way to measure your own blood sample from the comfort of your home. However, it won’t be as accurate as a blood pH test at your doctor’s office.

Urine pH Test
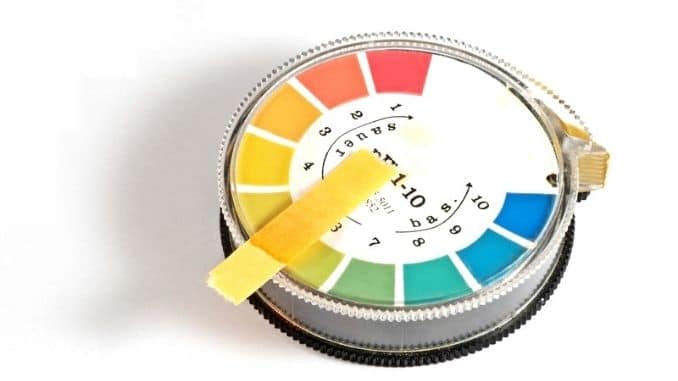
A urine pH level test analyzes the acidity or alkalinity of a urine sample. It’s a simple and painless test. A urine pH litmus paper test won’t show your blood’s pH level, but it may help show that something is off-balance.
What Affects the Body’s pH Level?
pH balance is one of the most important aspects of overall health, yet it’s something that many people don’t give enough attention to. pH levels affect everything from energy to weight loss, and yet many people still don’t know what affects the pH level of their body or how to adjust it. The list below can affect your pH balance:
Fluid loss. Losing too much water from your body or suffering from dehydration can increase your blood pH level. This happens when you also lose some blood electrolytes (salts and minerals, often sodium and potassium) with water loss. Common causes of fluid loss include excess sweating, vomiting, or diarrhea.
Diuretics. Diuretic drugs and other medications may cause you to urinate too much, leading to high blood pH levels. Treatment for fluid loss includes getting plenty of fluids and replacing electrolytes. Sports drinks can sometimes help with this. Your doctor may also stop any medications that cause fluid loss.
Kidney problems. Your kidneys help to keep your body’s acid-base balance. A kidney problem can lead to a high blood pH level. This may happen if the kidneys don’t remove enough alkaline substances through the urine. For example, bicarbonate may be incorrectly put back into the blood.
Diet. The food we eat has a large impact on our bodies’ pH levels, as well as how acidic or alkaline our stomach is. Foods such as meat (especially red meat) and other animal products have a high acid level, causing an increase in stomach acidity and subsequently an increase in the body’s overall pH level. On the other hand, foods like fruits and vegetables cause your stomach to produce less acid because they don’t require so much digestive work from your system, essentially creating a more alkaline environment within your body. It is interesting to note that fruits considered higher in acid, such as lemons, when mixed with water can have an alkalizing effect when digested.
Diabetic ketoacidosis. If you have diabetes, your blood may become acidic if your blood sugar levels aren’t properly managed.

What PRAL Is, and How You can Calculate It
Potential Renal Load (PRAL) is a measurement of the amount of acid your diet produces in your body. In general, foods rich in protein, such as meat, cheese and eggs, increase the production of acid in the body, whereas fruit and vegetables increase alkalis. Diets high in PRAL induce a low-grade metabolic acidosis state, which is associated with the development of metabolic alterations. The outcome of this alteration can include, insulin resistance, diabetes, hypertension, chronic kidney disease, bone disorders, low muscle mass and other complications.
Higher PRAL values indicate more acid is produced from that food we ingest, while negative PRAL values indicate that base is produced. PRAL is calculated from 5 nutrients that create either acid or base in our bodies. Protein and phosphorus create acid during metabolism, whereas potassium, magnesium and calcium produce base. By far, animal protein is the biggest determinant of PRAL; the higher the protein of a food, the higher the PRAL value.

Benefits of More Alkaline Diet
Alkaline diets reduce a person’s consumption of fatty and processed meats, and they encourage people to eat more fruits and vegetables. While the acidity/alkalinity of the foods we consume does have a direct effect on the pH of our urine, it does not impact the pH of the blood. The human body has many effective mechanisms set in place to closely regulate pH levels throughout the body, making it near impossible for outside influences to be of consequence. Basically, the body does a pretty good job of maintaining its own pH balance without us going on a diet to assist this. However, there can be many benefits to a more alkaline diet.
Promote Weight Loss. Ultimately, weight loss depends on consuming fewer calories than one burns. Diets lower in fat and calories may promote weight loss, but only when a person remains physically active and eats a healthful diet with variety. An alkaline diet tends to be low in calories, so it may help people lose weight.
Improving Kidney Health. For people with kidney disease, a lower-acid diet may improve symptoms or even slow the course of the disease.
Preventing Cancer. Significant evidence from a 2010 study suggests that reducing meat consumption and eating more fruits, vegetables, and whole grains might help prevent cancer.
Improving Joint Pain. Too much uric acid in your body can build up and cause tiny sharp crystals to form in and around joints. These crystals can cause the joints to become inflamed and painful. An alkaline diet balances your body’s pH, thus, reducing the production of uric acid.
Preventing Osteoporosis. Osteoporosis is a major risk factor for bone fractures, especially in older people and females. Some proponents of this diet say that it reduces the amount of calcium lost in urine, and that this lowers osteoporosis risk.

How Balanced pH can Help Athletes Recover
During exercise, the muscles use up oxygen as they convert glucose to energy. The production and removal of carbon dioxide and hydrogen, together with the use and transport of oxygen, cause chemical changes in the blood. These chemical changes, unless offset by other physiological functions, cause the pH of the blood to drop. If the pH of the body gets too low, acidosis results.
One of the main reasons athletes don’t perform as well as they should is because they don’t maximize recovery. Athletes tend to emphasize training, but not recovery. When an athlete has an optimum blood pH level, recovery is enhanced.
In conclusion, acid levels in your body can affect everything from your overall health and fitness level to how you feel emotionally. The acidity of your blood is a measure of how acidic or alkaline the solution in your bloodstream is, and it’s an important factor to consider when you’re trying to maintain good health. For example, high levels can lead to kidney stones and other serious problems that could affect a person for their entire life. On the flip side, low levels may increase risk factors for osteoporosis as well as cause chronic fatigue.
If you are an active person like an athlete, your blood needs to be at the right pH balance for optimal health and performance. It can also affect how well your body absorbs nutrients from food, so it’s important to maintain the proper acidity level in our bloodstreams by following some simple tips like drinking alkaline water, eating more fruits and vegetables, avoiding processed foods high in sugar content, limiting alcohol intake – especially beer which contributes to low pH levels and exercising regularly (which helps burn off acids).
By learning more about how to take care of your body you can really start understanding the balance that is beneficial for you and your needs. But for now, make sure you’re getting enough water! Have a glass of lemon-infused water before bed tonight and start thinking about incorporating more fresh fruit or vegetables into your diet this weekend. You deserve it!